
Effective advertising is essential for healthcare professionals and organizations to reach new patients and maintain trust with existing ones. However, healthcare advertising is subject to complex regulations and compliance requirements. These rules ensure the safety, privacy, and well-being of consumers, and navigating them can be a daunting task.
This article provides a comprehensive overview of healthcare advertising compliance and regulations. We will discuss the legal and ethical guidelines governing healthcare marketing, covering the roles of regulatory bodies, fundamental laws, and unique aspects of various healthcare sectors.
By equipping you with the necessary knowledge, you can ensure your advertising efforts are both effective and compliant, safeguarding your organization’s reputation and fostering trust with patients.
To effectively navigate the complex landscape of healthcare advertising compliance, it’s crucial to understand the foundations underpinning the various rules and regulations. This section will cover the roles of regulatory bodies and the key laws and regulations that govern healthcare advertising.
Several regulatory bodies oversees healthcare advertising compliance in the United States. The most prominent of these is the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC). Additionally, state-level agencies may have their own specific regulations that must be considered.
The FDA is responsible for regulating the advertising and promotion of prescription drugs, over-the-counter (OTC) drugs, and medical devices. The FDA’s primary goal is to ensure that healthcare advertising is truthful, balanced, and not misleading and that it conveys adequate information about a product’s safety and effectiveness.
The FTC’s role in healthcare advertising compliance is focused on preventing false and deceptive advertising practices for both OTC drugs and medical services provided by healthcare professionals. The agency enforces the Federal Trade Commission Act (FTC Act), which prohibits “unfair or deceptive acts or practices” in commerce, including advertising.
In addition to federal regulations, healthcare advertising is often subject to state-level oversight. Each state may have its own laws and agencies responsible for regulating advertising practices within its borders. It’s important for healthcare organizations to be aware of and comply with state-specific regulations.
Healthcare advertising compliance is shaped by several laws and regulations that govern different aspects of the industry. Some of the most significant laws and regulations include:
Understanding the roles of regulatory bodies and the key laws and regulations that govern healthcare advertising is fundamental to ensuring compliance and avoiding legal or financial consequences.
In addition to the legal framework governing healthcare advertising, ethical considerations play a significant role in shaping responsible and effective marketing practices. This section will discuss the importance of providing truthful and non-misleading information, maintaining patient privacy and confidentiality, and embracing cultural sensitivity and diversity in healthcare advertising.
Healthcare organizations and professionals have an ethical obligation to provide accurate, truthful, and non-misleading information in their advertising. This not only helps to maintain trust with patients but also ensures that healthcare consumers can make informed decisions about their care.
To maintain ethical standards in healthcare advertising, it’s crucial to verify that all claims made in marketing materials are accurate and supported by scientific evidence. This includes a proper citation of relevant studies, as well as presenting a balanced view of potential benefits and risks associated with a product or service.
Deceptive advertising practices, such as making unsubstantiated claims or using misleading imagery, can erode patient trust and harm public health. Healthcare organizations should avoid these tactics and commit to transparent, honest communication in their advertising.

Healthcare advertising must respect patient privacy and adhere to strict confidentiality guidelines, particularly when it comes to the use of patient information and testimonials.
HIPAA and other privacy regulations require healthcare organizations to obtain proper authorization and follow strict protocols when handling protected health information (PHI) in advertising. This includes de-identifying patient information and ensuring that privacy is maintained at all times.
As mentioned earlier, HIPAA plays a crucial role in safeguarding patient privacy. Healthcare organizations must familiarize themselves with the specific provisions of HIPAA that apply to advertising and ensure their marketing materials are fully compliant.
If you’d like a more in-depth understanding of HIPAA, be sure to visit the U.S. Department of Health & Human Services website. They offer a comprehensive summary that covers essential aspects of this crucial regulation, ensuring you’re well-informed and knowledgeable about its implications.
In today’s diverse society, it’s essential for healthcare advertising to be culturally sensitive and inclusive, addressing the needs and concerns of different communities.
Healthcare organizations should strive to create advertising that reflects the diversity of the communities they serve. This includes using inclusive language, imagery, and messaging that acknowledges the unique needs and perspectives of various cultural and demographic groups.
To maintain ethical standards in healthcare advertising, it’s important to avoid perpetuating stereotypes or biases. Instead, organizations should strive for inclusivity and cultural competence, fostering a sense of belonging and trust among diverse patient populations.
By embracing ethical considerations in healthcare advertising, organizations can foster trust with patients, ensure compliance with relevant regulations, and promote informed decision-making among healthcare consumers.
Pharmaceutical advertising is a critical component of the healthcare industry, as it informs patients and healthcare providers about the latest medications and their potential benefits. However, it’s also subject to stringent regulations to ensure that consumers receive accurate and balanced information. This section will discuss the key aspects of pharmaceutical advertising, focusing on prescription drug advertising and over-the-counter (OTC) drug advertising.
Prescription drug advertising is a highly regulated area within the healthcare industry, as it plays a significant role in informing patients and healthcare providers about the potential benefits, risks, and proper use of medications. The Food and Drug Administration (FDA) is the primary regulatory body responsible for overseeing prescription drug advertising in the United States, with a focus on ensuring that advertisements provide accurate, balanced, and non-misleading information to consumers.
The FDA’s oversight of prescription drug advertising involves several key components:
If you’re looking for more information on pharmaceutical advertising, be sure to check out the helpful FAQs provided by the FDA. They offer valuable insights and guidance on this important topic, so you can stay informed and up-to-date.
Over-the-counter (OTC) drug advertising plays a vital role in informing consumers about the availability, benefits, and proper use of non-prescription medications. The Federal Trade Commission (FTC) is the primary regulatory body responsible for overseeing OTC drug advertising in the United States. The FTC enforces guidelines aimed at preventing false and deceptive advertising practices and ensuring that consumers receive accurate, reliable, and transparent information about OTC products.

The FTC has established specific guidelines for OTC drug advertising to ensure that all promotional materials are truthful, non-misleading, and based on competent and reliable scientific evidence. These guidelines encompass several key principles:
For additional resources on over-the-counter drug advertising, don’t forget to explore the informative press releases provided by the FTC.
Compliance with FTC guidelines is crucial for OTC drug advertisers to maintain trust with consumers and promote the responsible use of medications. Key strategies for ensuring safety and efficacy in OTC drug advertising include:
By following the FTC’s guidelines for OTC drug advertising, pharmaceutical firms can establish credibility with consumers, facilitate informed decision-making, and promote the responsible consumption of over-the-counter medications.
Medical device advertising is essential for raising awareness among healthcare professionals and patients about new technologies and treatments. Like pharmaceutical advertising, medical device advertising must adhere to stringent regulations to ensure that the information provided is accurate, balanced, and non-misleading. This section will delve into the regulatory landscape of medical device advertising, highlighting the FDA’s role, unique considerations for different device classifications, and the importance of substantiating claims with scientific evidence.
The FDA plays a critical role in regulating medical device advertising in the United States. Its regulations are designed to ensure that promotional materials meet specific standards of accuracy and clarity.
The FDA’s oversight ensures that all claims made in promotional materials are truthful, non-misleading, and supported by substantial evidence. This includes verifying that the device’s benefits, risks, and indications for use are accurately and fairly represented in the advertisement.
Actively monitoring medical device advertisements for compliance with its regulations, the FDA investigates potential violations and takes necessary enforcement action. This process may involve issuing warning letters, seeking injunctions to halt the dissemination of non-compliant advertisements, or implementing other remedies to protect public health.
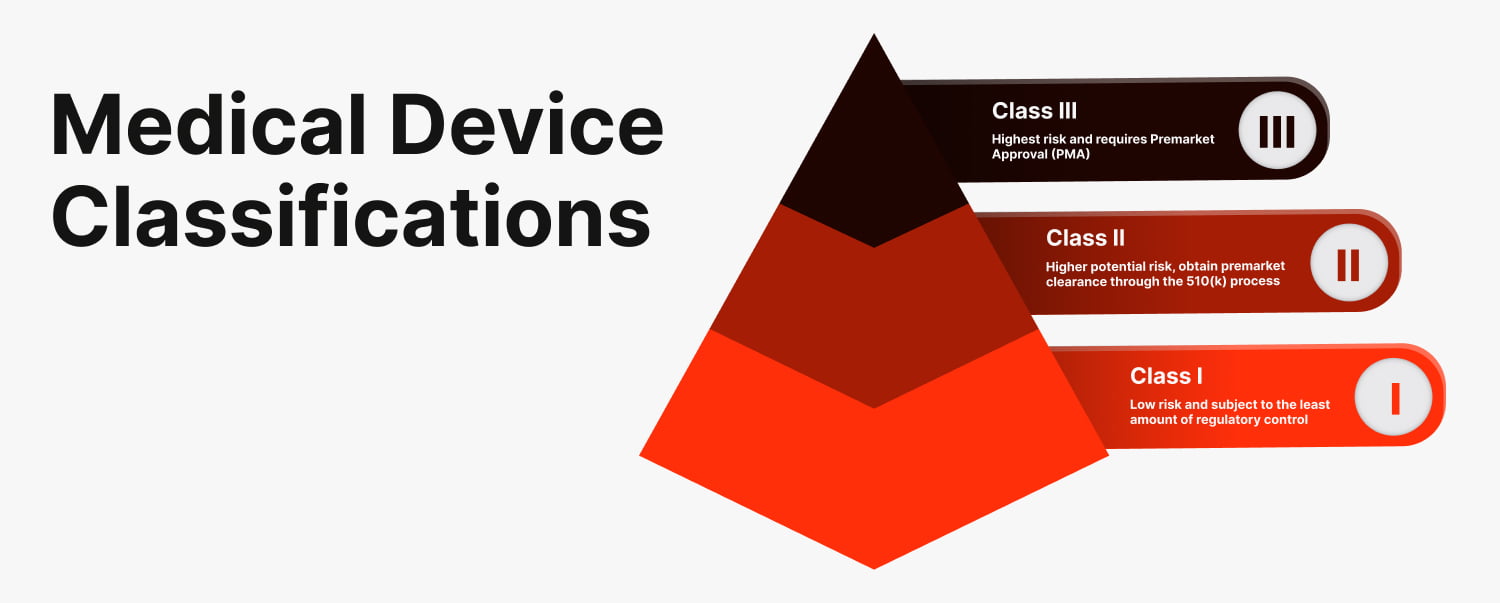
The FDA classifies medical devices into three categories, depending on their potential risk to patients and the level of regulatory control required to ensure their safety and effectiveness. We will give you a summary of each classification, however, we recommend that you expand your knowledge by viewing the FDA’s regulatory controls.
In medical device advertising, substantiating claims with robust scientific evidence is crucial to ensuring that healthcare professionals and patients receive accurate and reliable information. This section emphasizes the key aspects of validating claims and presenting data transparently in medical device advertisements.
To uphold the credibility of their claims, medical device advertisers must conduct well-designed, controlled clinical studies that demonstrate the safety and effectiveness of their devices. These studies should adhere to FDA guidelines and provide ample evidence to support the claims made in promotional materials.
Besides offering scientific evidence to back up their claims, medical device advertisers must also present this data in a clear and easily understandable manner. By doing so, they can ensure transparency and support informed decision-making among healthcare professionals and patients. Key aspects of transparent data presentation include:
By upholding these principles and validating claims through scientific evidence, medical device manufacturers can foster trust among healthcare professionals and patients, guaranteeing access to precise, dependable information regarding novel technologies and therapies.
The evolving digital landscape presents unique challenges and opportunities for healthcare advertisers. This section delves into various aspects of digital healthcare advertising, focusing on platform-specific regulations, data privacy, and best practices for optimizing online advertisements.
Different online platforms have their own specific regulations and guidelines for healthcare advertising. Advertisers must be aware of these requirements to ensure compliance and maintain a strong online presence.
For instance, Google Ads has strict policies for healthcare and medicines advertising, which vary by country. Advertisers must follow these policies, including restrictions on certain drug types and advertising techniques, to avoid disapproval or suspension of their ads.
Similarly, Facebook and Instagram have guidelines in place for healthcare advertising. Advertisers must comply with Facebook’s advertising policies, which cover issues such as targeting, prohibited content, and data usage, to run successful campaigns on the platform.
As a professional networking site, LinkedIn also has its own set of advertising policies that healthcare advertisers must adhere to, including restrictions on targeting options and requirements for ad creatives.
To learn more about specific advertising platforms and channels check out our free resource.
Navigating various data protection regulations is crucial for healthcare advertisers advertising online. The Health Insurance Portability and Accountability Act (HIPAA) regulates the use and disclosure of protected health information (PHI) in the United States. Healthcare advertisers must ensure that their online advertising strategies comply with HIPAA privacy and security rules.
The General Data Protection Regulation (GDPR) is a comprehensive data protection law that affects businesses operating within the European Union (EU) or targeting EU citizens. Advertisers must be aware of the GDPR’s requirements, including obtaining user consent for data processing and providing users with the right to access, correct, or delete their personal data.
To effectively engage and inform audiences in the digital space, healthcare advertisers must follow best practices for creating compelling, informative, and compliant online advertisements.
By understanding the unique challenges and opportunities of digital healthcare advertising, advertisers can successfully navigate the online landscape, reaching their target audiences while remaining compliant with relevant regulations and guidelines.

Navigating the world of healthcare provider advertising can be complex, but understanding the best practices and guidelines can help ensure a successful marketing strategy. In this section, we will explore key considerations, ethical concerns, and the importance of transparency in healthcare provider advertising.
When advertising their services, healthcare providers should keep certain factors in mind to ensure they deliver accurate and helpful information to potential patients. Understanding the needs and preferences of your target audience will enable you to craft messages that resonate with them and address their concerns effectively. It’s also essential to showcase your professional credentials, experience, and unique services to build trust and credibility with potential patients. Lastly, familiarize yourself with state and federal regulations governing healthcare provider advertising to avoid potential legal issues.
Ethics play a crucial role in healthcare provider advertising. Providers should prioritize patient well-being and avoid engaging in misleading or deceptive practices. Ensure that all claims made in your advertisements are truthful, accurate, and backed by evidence. Additionally, be cautious about sharing patient testimonials or success stories, ensuring that you have obtained proper consent and are not disclosing any confidential information.
Maintaining transparency in your advertising efforts is key to building trust and fostering a positive relationship with potential patients. Provide accurate information about the costs of your services and the insurance plans you accept to help patients make informed decisions. Inform patients about any potential risks, complications, or side effects associated with the treatments and procedures you offer.
By considering these best practices and guidelines, healthcare providers can create effective advertising strategies that convey their expertise and commitment to patient care while maintaining ethical standards and transparency.
At Webserv, a top healthcare marketing agency, we specialize in crafting tailored, compliance-focused advertising strategies that drive results for your medical practice. Our expertise in Paid Media and commitment to navigating the complex landscape of healthcare advertising compliance and regulations ensure your campaigns stay within the legal boundaries while resonating with your target audience.
Book a meeting with our expert team today and experience the benefits of partnering with Webserv, where compliance, marketing success, and cutting-edge Paid Media strategies go hand in hand.
Non-compliant healthcare advertising can result in warning letters, fines, penalties, legal actions, and damage to the organization’s reputation and patient trust.
Healthcare companies can ensure compliance by staying informed about regulations, working with legal and regulatory experts, implementing thorough review processes, and collaborating with specialized healthcare marketing agencies like Webserv.
Healthcare providers should be aware of HIPAA compliance, adherence to ethical guidelines, state-specific regulations, and the importance of transparency, accuracy, and non-misleading communication in advertising.
Online healthcare advertising follows the same regulations as traditional methods but has additional guidelines for platform-specific policies, targeting, monitoring, and proper disclosure mechanisms in digital spaces.
A healthcare marketing agency, like Webserv, offers specialized expertise in regulatory compliance, helping organizations develop and execute advertising strategies that adhere to guidelines, mitigate risks, and effectively reach target audiences while maintaining a strong reputation and fostering patient trust.
